
Meaningful Use Reporting
Meaningful Use – Cancer Reporting in California
The California Department of Public Health (CDPH) is coordinating all reporting for Meaningful Use(MU) for California. CDPH is utilizing a Health Information Exchange (HIE) Gateway to receive and process MU files. For more information on the CDPH HIE Gateway, please go to Health Information Exchange Gateway website.

To participate in MU2 for Cancer Reporting, Physician Offices (PO) must meet the HL7 Clinical Document Architecture (CDA) 2.0 standard, and messages must conform to the CDA implementation specifications found in the Implementation Guide for Ambulatory Healthcare Provider Reporting to Central Cancer Registries. In addition, the technology used by the PO to generate the HL7 CDA. XML message has to be a Federally Certified 2014 Edition EHR technology.
There are two transmission options for submitting MU2 data for Cancer Reporting:
- File Upload via Hypertext Transfer Protocol Secure (HTTPS)
- Secure File Transfer Protocol (SFTP)
If you do not know your transport method, please ask your Information Technology representative or your Electronic Health Record vendor prior to enrolling to submit MU2 cancer data.
Meaningful Use Stage 3
The California Cancer Registry is declaring its readiness to meet the Stage 3 Meaningful Use Cancer Measure for early adopters of Stage 3 Meaningful Use in 2017. CCR will be able to meet the 2015 Edition CEHRT criteria, including incorporation of the updated Cancer Implementation Guide
HL7 CDA ® Release 2 Implementation Guide: Reporting to Public Health Cancer Registries from Ambulatory Healthcare Providers, Release 1, DSTU Release 1.1 – US Realm
Please Note:
The current Meaningful Use system does not meet cancer reporting requirements. If you participate in Meaningful Use, you must still report cancer cases directly to your cancer registry.
———————————————————————————————————————————————–
Steps for MU Reporting Through CDPH
STEP 1: Begin the online registration process
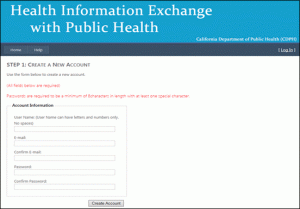
STEP 2: Create an account
Follow the on-screen directions to create a new account and click “create account.”
STEP 3: Receive email confirmation from HIE
You will receive an email from HIEHelp@cdph.ca.gov, requesting confirmation of your registration. Click on the link provided in the email.
STEP 4: Enter contact information
Enroll for cancer reporting by filling in your contact information.

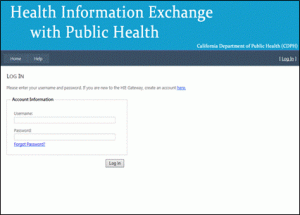
STEP 5: Log-in page
You will automatically be redirected to the log-in page. You will be directed to create an account by clicking on a link.
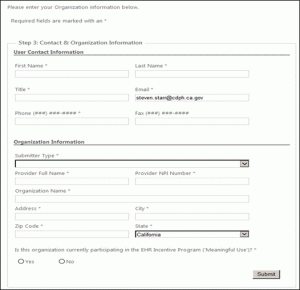
STEP 6: Enter your information
You will be redirected to a page to enter your information as shown below. To proceed, complete the minimum fields, as indicated by asterisks and click “submit.”
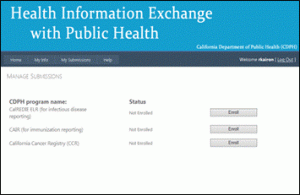
STEP 7: Manage Submissions page
You will be redirected to the Manage Submissions page as shown below. Click “Enroll” for the California Cancer Registry (CCR).
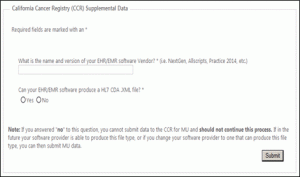
STEP 8: Supplemental Data page
You will be redirected to the CCR Supplemental Data page. Answer the questions and click submit.
STEP 9: MU enrollment is pending
The following screen will appear to indicate your MU enrollment application is pending.

STEP 10: Enrollment confirmation
Next, you will receive an email from CDPH HIE Gateway, letting you know that you have successfully enrolled with CCR. The email will provide you with directions and options for transmitting your data.
STEP 11: Testing and validation
After registering, you must complete the testing and validation process. You will need to decide how you want to submit your MU cancer data. There are 2 options available to you that will allow the data to go directly to CCR:
After selecting your transmission option, you may submit HL7 CDA.XML files to CCR. For the Testing & Validation phase, you will be asked to submit 5 test messages that pass the CDC Validation process. Once your office has achieved this, you will then be moved to Production for ongoing data submission.
STEP 12: Ongoing data submission
You can begin ongoing data submission in compliance with MU for cancer reporting. While actively engaged in the steps above, you will be receiving emails and possibly telephone calls from CDPH HIE and CCR. You should maintain copies of these communications for EHR Incentive Program audit purposes.
If you have any questions regarding these processes, please contact our office at MU2CCRHelp@ccr.ca.gov.
More Information about MU Reporting
For more information about MU reporting, see the FAQ provided by CDPH.

